Recent amendments to the Clean Air Act require use of oxygenated gasoline during winter months in about forty urban areas across the United States. Generally, winter gasoline must contain 2.7 percent by weight of oxygen; California has been allowed to use a lower level, 2.0 percent. To determine the effectiveness of oxygenated gasoline in reducing carbon monoxide (CO) emissions, we designed a study to expand upon previous emission research by testing a large number of on-road vehicles.
States. Generally, winter gasoline must contain 2.7 percent by weight of oxygen; California has been allowed to use a lower level, 2.0 percent. To determine the effectiveness of oxygenated gasoline in reducing carbon monoxide (CO) emissions, we designed a study to expand upon previous emission research by testing a large number of on-road vehicles.
Testing Oxygenated Gasoline in the Field
The federal government relies on a traditional testing method using a dynamometer to measure vehicle emissions. Testers keep vehicles stationary while running their engines and simulating standard stop-and-go city driving conditions. Since this procedure is time-consuming and expensive, the government could not feasibly test large numbers of vehicles. Therefore, the sample sizes studied may have been too small to represent the range of vehicles actually on the road.
 To put oxygenated gasoline to a real-life test, two graduate students, Tom Kirchstetter and Brett Singer, and I measured vehicle emissions in the Caldecott Tunnel, located on Highway 24 between Oakland and Orinda, California. In cooperation with the Bay Area Air Quality Management District, we measured pollutant concentrations of exhaust samples drawn through slots in the ventilation system of the tunnel. By using the central bore of the three-bore tunnel, where no heavy-duty trucks are allowed, we could limit our study to light-duty vehicles. Over 4,000 vehicles pass through this section every hour during the afternoon rush-hour sampling period.
To put oxygenated gasoline to a real-life test, two graduate students, Tom Kirchstetter and Brett Singer, and I measured vehicle emissions in the Caldecott Tunnel, located on Highway 24 between Oakland and Orinda, California. In cooperation with the Bay Area Air Quality Management District, we measured pollutant concentrations of exhaust samples drawn through slots in the ventilation system of the tunnel. By using the central bore of the three-bore tunnel, where no heavy-duty trucks are allowed, we could limit our study to light-duty vehicles. Over 4,000 vehicles pass through this section every hour during the afternoon rush-hour sampling period.
We took samples on ten days in August 1994 and ten days in October 1994. In California, gasoline must be oxygenated from October 1 to January 31. The California Air Resources Board collected and analyzed gasoline samples from Bay Area service stations during our two sampling periods at the Caldecott Tunnel. The measured oxygen content in gasoline increased from 0.3 percent by weight in August to 2.0 percent by weight in October. Measured ambient air temperatures at the Tunnel differed by only one or two degrees Celsius between August and October. Traffic leaving the Tunnel was monitored to determine traffic flow, proportion of cars and trucks, and average speed. As expected, the fraction of heavy-duty diesel trucks in the center bore of the Tunnel was small (less than 0.2 percent), so the measurements in the Tunnel reflect emissions from gasoline-powered cars and light trucks.
Our results showed a CO decrease of about 21 (±7) percent and a volatile organic compound (VOC) emissions decrease of about 18 (±10) percent following the introduction of oxygenated gasoline. However, no significant change was found for emissions of oxides of nitrogen (NOx).
Field measurements taken in the Caldecott Tunnel reflect emissions from vehicles operating in hot stabilized mode. An important issue that 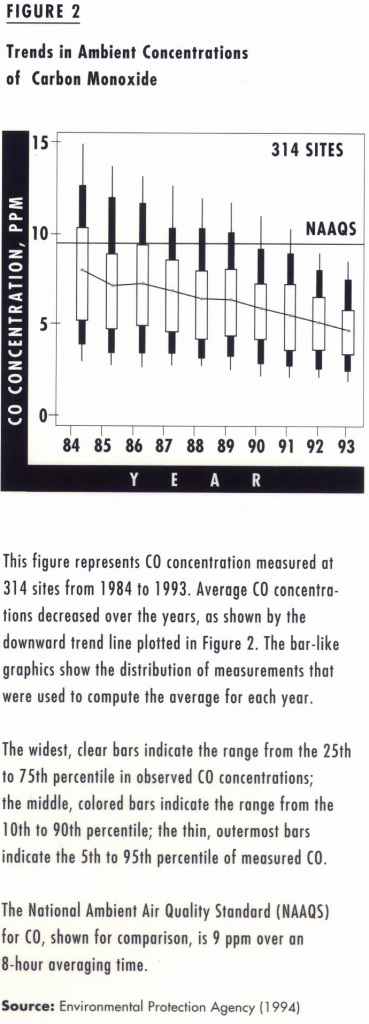 requires further study is the effect of oxygenated gasoline on cold start emissions. Vehicle exhaust emissions are especially high during the first few minutes of operation when the engine and emission control systems are still cold.
requires further study is the effect of oxygenated gasoline on cold start emissions. Vehicle exhaust emissions are especially high during the first few minutes of operation when the engine and emission control systems are still cold.
We had mixed results for certain individual hazardous organic species that are part of total VOC emissions. Following the introduction of oxygenated gasoline, measured emissions of benzene, a known human carcinogen, decreased by about 25 (±17) percent, while emissions of formaldehyde, a respiratory and ocular irritant, increased by about 13 (± 6) percent.
Carbon Monoxide’s Decreasing Significance as a Major Air Pollutant
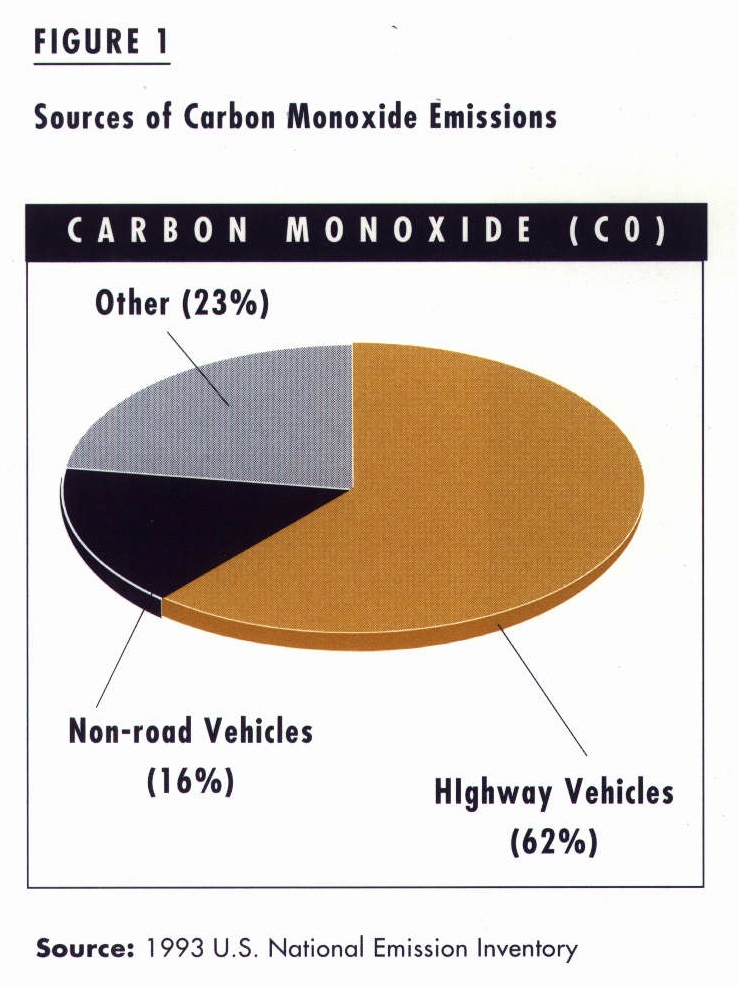
While motor vehicles continue to produce the greatest proportion of CO emissions nationwide (see Figure 1), along with significant amounts of VOC and NOx, ambient carbon monoxide concentrations are dropping rapidly (see Figure 2). These reductions, which are mainly due to stricter emission standards for new cars and trucks, are occurring despite increasing numbers of vehicles on the road. If we continue using oxygenated gasoline and retiring older vehicles, carbon monoxide will soon cease to be a major air pollution problem in outdoor air.
It is therefore surprising that carbon monoxide emissions still receive so much attention in transportation planning and motor vehicle emission-control efforts. California’s air quality impact assessments hinge on dispersion modeling for carbon monoxide. Although these assessments should remain an important element of transportation systems design, the assessment should now focus on other emissions.
The Real Threats to Human Health
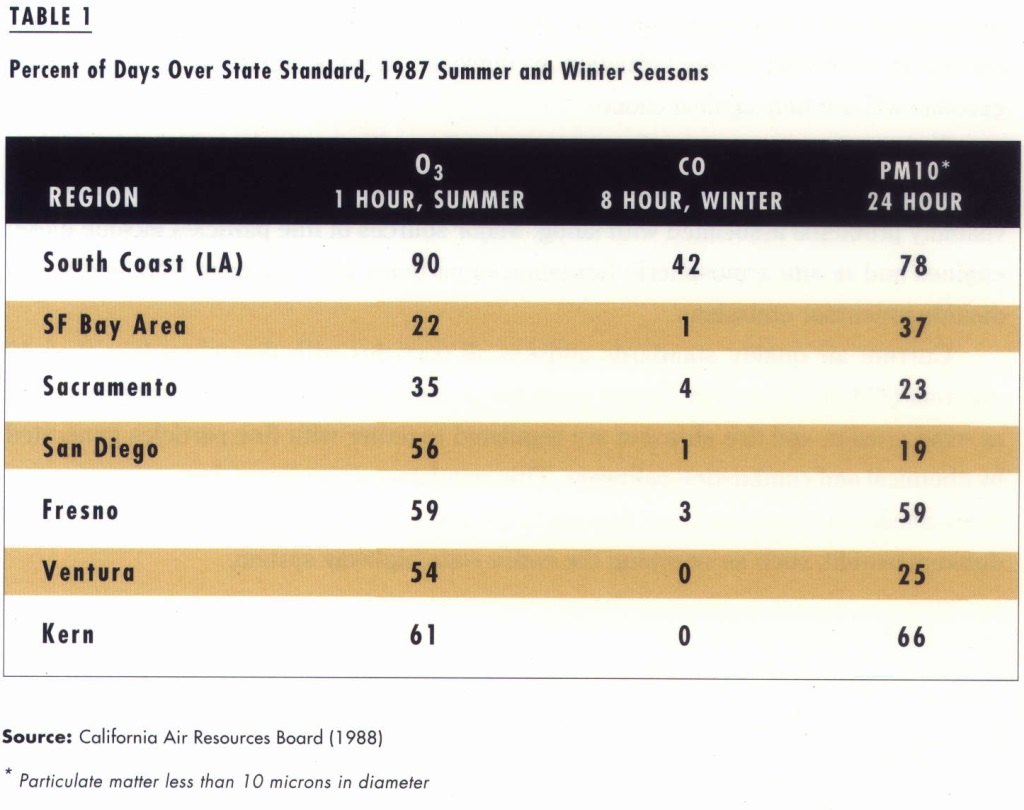
Ozone and airborne particulate matter pose a greater problem than CO. For example, in 1987, all regions in California exceeded air quality standards for those pollutants more often than for CO (see Table 1).
Ozone is not emitted directly, but formed in the lower atmosphere by reactions involving VOC and NOx emissions. As of 1989, ninety-six metropolitan areas nationwide did not meet the statutory ambient air quality standards for ozone. Oxygenated gasoline may help against ozone in some, but not all, areas. In locations such as Los Angeles and San Francisco, where VOC emissions are low, oxygenated gasoline may help reduce ozone formation. In locations such as Atlanta, where natural VOC emissions from vegetation are very high, ozone formation is controlled by NOx emissions, so oxygenated gasoline will not help against ozone.
Perhaps the most serious threat to current air quality comes from fine airborne particles (those with diameters less than 2 microns), which cause the brownish haze and visibility problems associated with smog. Major sources of fine particles include diesel engines and in situ atmospheric formation of particles from gaseous NOx and sulfur dioxide precursor emissions.
Current air quality standards address all particles with diameters less than 10 microns (PM10). Therefore, larger particles generated by mechanical processes such as wind erosion and tire abrasion are regulated together with fine particles generated by chemical and combustion  pathways. This regulatory grouping of fine and coarse particles masks the importance of fine particles and encourages air control measures of dubious benefit, such as sweeping the entire state highway system.
pathways. This regulatory grouping of fine and coarse particles masks the importance of fine particles and encourages air control measures of dubious benefit, such as sweeping the entire state highway system.
Finally, by requiring costly oxygenated gasoline, we are placing a financial burden on all motorists when only ten percent of cars and trucks contribute fifty percent or more of all vehicle emissions. New cars, with effective emission control systems, no longer contribute significantly to pollution.
Our study shows that oxygenated gasoline effectively reduces CO emissions. Moreover, it emphasizes an important fact: CO is no longer a problem in outdoor air. Current research and legislation should focus on ozone and fine particles. The success of oxygenated gasoline must not foster complacency about overall motor vehicle emissions. Instead, it should be viewed as a first step toward creating a non-polluting fuel for the next century.
Further Readings
J.G. Calvert, J.B. Heywood, R.F. Sawyer, and J.H. Seinfeld, ” Achieving Acceptable Air Quality: Some Reflections on Controlling Vehicle Emissions,” Science, Vol. 261, 1993, pp. 37-4S.
Environmental Protection Agency, National Air Quality and Emissions Trends Report, Office of Air Quality Planning and Standards, EPA-454/R-94-026 (Research Triangle Park, North Carolina, 1994).
T.W. Kirchstetter, B.C. Singer, R.A. Harley, G.R. Kendall, and W. Chan, “Impacts of Oxygenated Gasoline Use on California Light-Duty Vehicle Emissions,” forthcoming in Environmental Science & Technology, 1995.
National Research Council, Rethinking the Ozone Problem in Urban and Regional Air Pollution (Washington, DC: National Academy Press, 1991).
C.A. Pope, M.J. Thun, M.M. Namboodiri, D.W. Dockery, J.S. Evans, F.E. Speizer, and C.W. Heath, “Particulate Air Pollution as a Predictor of Mortality in a Prospective Study of U.S. Adults,” American Journal of Respiratory and Critical Care Medicine, Vol. 151, 1995, pp. 669-674.